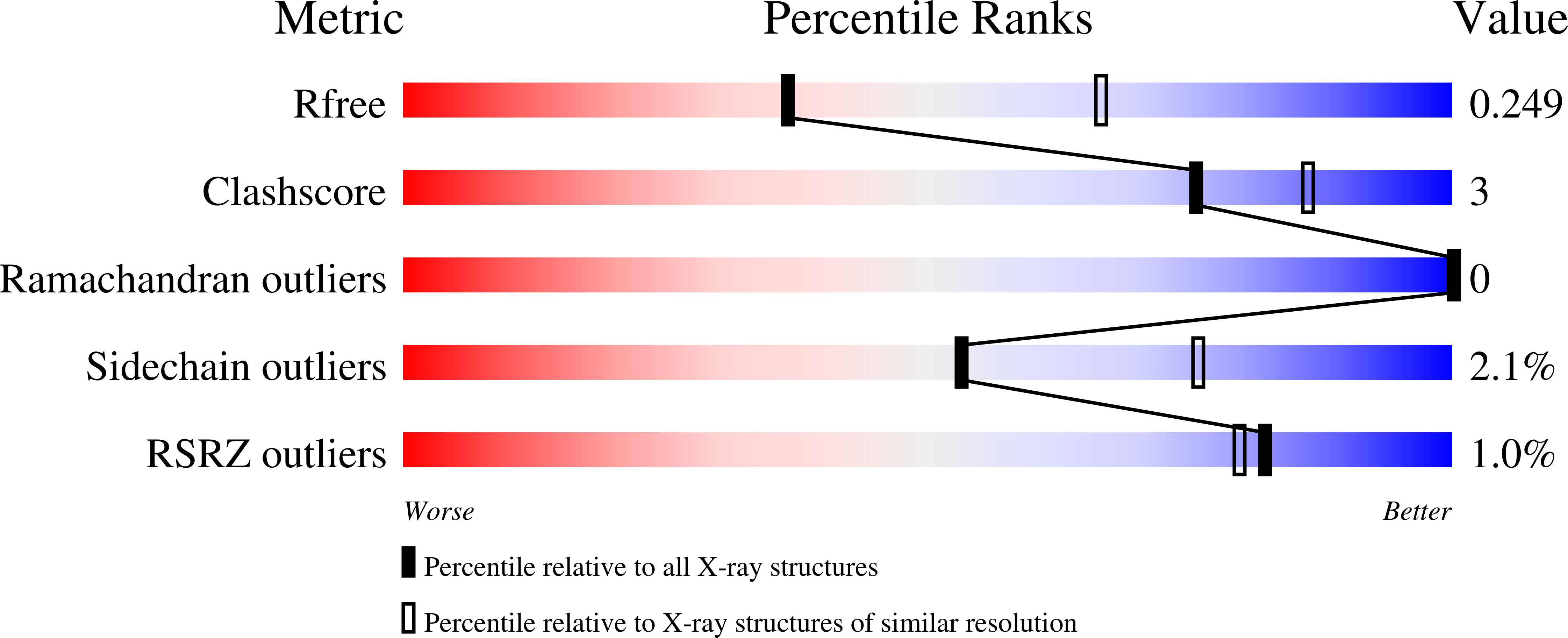
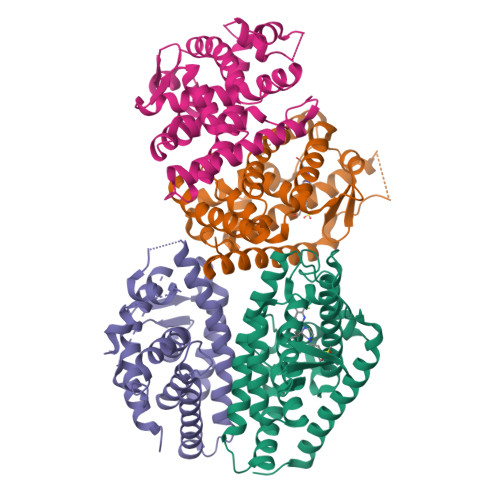
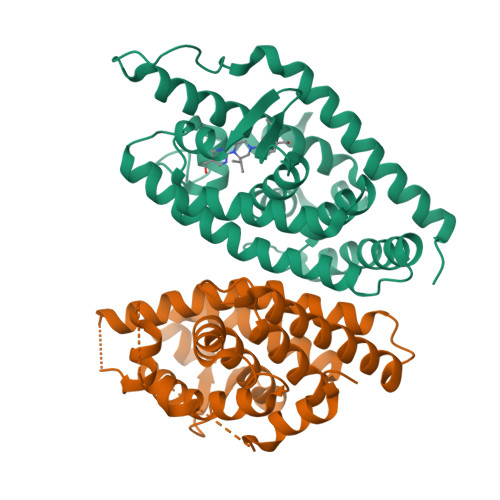
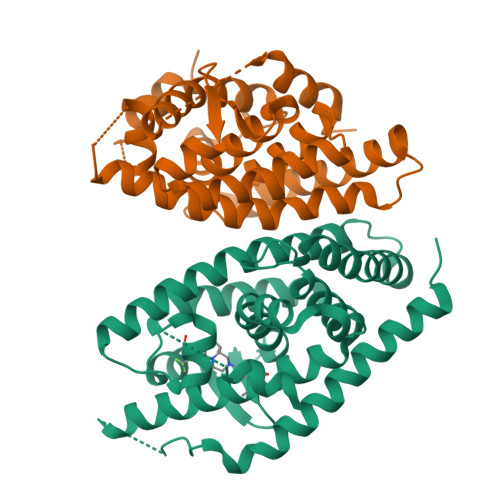
Discovery of a Novel, Orally Efficacious Liver X Receptor (LXR) beta Agonist.
Zheng, Y., Zhuang, L., Fan, K.Y., Tice, C.M., Zhao, W., Dong, C., Lotesta, S.D., Leftheris, K., Lindblom, P.R., Liu, Z., Shimada, J., Noto, P.B., Meng, S., Hardy, A., Howard, L., Krosky, P., Guo, J., Lipinski, K., Kandpal, G., Bukhtiyarov, Y., Zhao, Y., Lala, D., Van Orden, R., Zhou, J., Chen, G., Wu, Z., McKeever, B.M., McGeehan, G.M., Gregg, R.E., Claremon, D.A., Singh, S.B.(2016) J Med Chem 59: 3264-3271
- PubMed: 26990539
- DOI: https://doi.org/10.1021/acs.jmedchem.5b02029
- Primary Citation of Related Structures:
5I4V - PubMed Abstract:
This article describes the application of Contour to the design and discovery of a novel, potent, orally efficacious liver X receptor β (LXRβ) agonist (17). Contour technology is a structure-based drug design platform that generates molecules using a context perceptive growth algorithm guided by a contact sensitive scoring function. The growth engine uses binding site perception and programmable growth capability to create drug-like molecules by assembling fragments that naturally complement hydrophilic and hydrophobic features of the protein binding site. Starting with a crystal structure of LXRβ and a docked 2-(methylsulfonyl)benzyl alcohol fragment (6), Contour was used to design agonists containing a piperazine core. Compound 17 binds to LXRβ with high affinity and to LXRα to a lesser extent, and induces the expression of LXR target genes in vitro and in vivo. This molecule served as a starting point for further optimization and generation of a candidate which is currently in human clinical trials for treating atopic dermatitis.
Organizational Affiliation:
Vitae Pharmaceuticals, Inc. , 502 W. Office Center Drive, Fort Washington, Pennsylvania 19034, United States.